Abstract
BACKGROUND:
Myelosuppressive hematological adverse events (anemia, neutropenia, and thrombocytopenia) are common complications of chemotherapy among cancer patients. Cytotoxic chemotherapy remains the cornerstone of treatment for extensive stage small cell lung cancer (ES-SCLC). This study assessed the health care resource utilization (HCRU), costs, and treatment patterns associated with myelosuppressive hematological adverse events (HAEs) among ES-SCLC patients treated with chemotherapy in the community oncology setting.
METHODS:
A retrospective observational study was conducted using The US Oncology Network iKnowMed electronic health records data from 1/1/2015 to 12/31/2020. Adult patients with ES-SCLC who initiated chemotherapy (chemotherapy alone or chemotherapy in combination with immunotherapy) between 1/1/2015 and 12/31/2019 were identified and further stratified into two study cohorts: with grade ≥3 HAE vs. without grade ≥3 HAE after chemotherapy initiation. Date of chemotherapy initiation was considered as the index date. Patients were followed longitudinally from index date until 12/31/2020, death or last patient record, whichever came first. HAEs were identified using iKnowMed structured data for laboratory values based on Common Terminology Criteria for Adverse Events v5.0 definitions for anemia, neutropenia, and thrombocytopenia. Health care resource utilization and outpatient costs, and treatment patterns were evaluated for both cohorts. Inpatient costs or costs for transfusions were not included due to data limitation.
RESULTS:
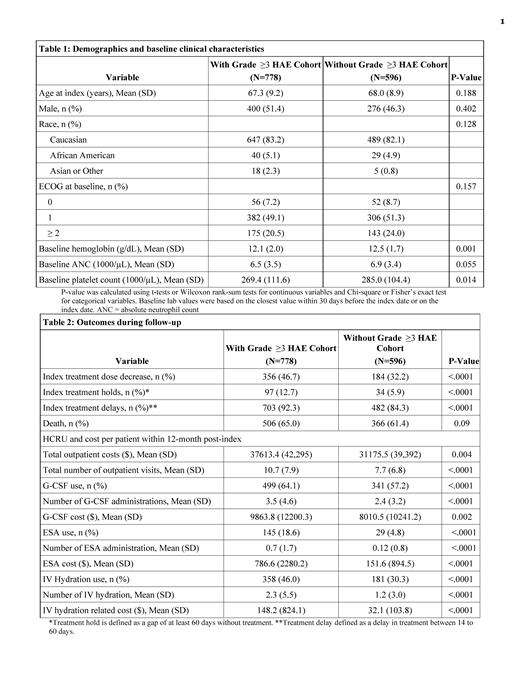
778 patients had at least one grade ≥3 HAEs after the chemotherapy initiation (50.3% with grade 3 anemia, 46.0% with grade 3 neutropenia, 28.0% with grade 4 neutropenia, 33.8% with grade 3 thrombocytopenia, 18.1% with grade 4 thrombocytopenia). Among the 778 patients, 454 (58.4%) of patients had grade ≥3 HAEs in 2 or more lineages (21.3% for anemia and neutropenia, 21.0% for neutropenia and thrombocytopenia, 20.6% for anemia and thrombocytopenia, and 12.2% for all 3 lineages). 43.1% of patients were eligible for RBC transfusions and 3.9% eligible for platelet transfusions as indicated by lab value. 12.2% of patients had evidence of major bleeding events. 596 patients were included in the without grade ≥3 HAE cohort. Demographics and baseline ECOG scores were similar for the two cohorts (Table 1). Almost all patients (>99%) received first-line chemotherapy at index (approximately 80% received platinum/etoposide regimen, 15% received platinum/etoposide in combination with immunotherapy) in both cohorts. Patients with grade ≥3 HAE had higher proportion of dose reductions (46.7% vs. 32.2%), treatment holds (12.7% vs. 5.9%), and treatment delays (92.3% vs. 84.3%) after chemotherapy initiation, all p<0.001 when compared to the patients without grade ≥3 HAE.
The total outpatient costs within 12 months post-index were higher for patients with grade ≥3 HAE ($37,613 vs. $31,176 p=0.004) (Table 2). Patients with grade ≥3 HAE had an average of 10.7 outpatient visits within 12 months post-index, vs 7.7 outpatient visits for those without grade ≥3 HAE (p<0.0001). Patients with grade ≥3 HAE also had higher G-CSF use (64.1% vs. 57.2%, mean number of administrations 3.5 vs 2.4, mean cost per patient $9864 vs. $8011, all p<0.01), ESA use (18.6% vs. 4.8%, 0.7 vs 0.12 administrations, mean cost per patient $786 vs. $151, all p<0.01), and IV hydration (46.0% vs. 30.3%, 2.3 vs 1.2 administrations, mean cost per patient $148 vs $32, all p<0.01) compared to the patients without grade ≥3 HAE.
CONCLUSIONS:
The results suggest there is significant burden of myelosuppressive hematological adverse events among ES-SCLC patients in a community oncology setting. A notable proportion of patients had HAEs in 2 or more lineages. Patients with grade ≥3 HAE appear to have more dose reductions, treatment delays, and more health care service utilizations than those without. Therapies to protect bone marrow from multilineage HAEs have potential to reduce such burden. Future research should investigate HCRU and cost burden in the inpatient setting to better understand the full scope of HAE management.
Goldschmidt: Ontada: Current Employment; Blue Ridge Cancer Care: Current Employment; Amgen: Honoraria, Speakers Bureau; BMS: Honoraria, Speakers Bureau; G1 Therapeutics: Honoraria, Speakers Bureau; TG Therapeutics: Honoraria. Monnette: Ontada: Current Employment, Current holder of individual stocks in a privately-held company; G1 Therapeutics: Consultancy; BMS: Consultancy. Shi: Ontada: Current Employment. Venkatasetty: Ontada: Current Employment. Huang: G1 Therapeutics: Current Employment, Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company. Chioda: G1 Therapeutics: Current Employment, Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal